
In rare disease research, collaboration is often the catalyst that transforms a promising idea into a life-changing treatment.
It’s this approach that allows us to develop life-changing medicines to treat rare diseases. As NS Pharma Vice President, Innovation Research Partnering Tatsuaki Morokata, Ph.D., puts it, “Collaborating with others acts as an accelerating system. A good collaboration enables us to do together what otherwise cannot be done.”

Tatsuaki Morokata, PhD, MBA
Vice President, Innovation Research Partnering, NS Pharma
Our expertise in pharmacology, clinical development, and regulatory affairs enables us to swiftly progress therapies from concept to clinic. Now, through our Innovation Research Partnering division, we’re forming strategic alliances with cutting-edge companies to turn pioneering science into real-world solutions.
One such partnership is with MiNA Therapeutics, the world leader in RNA activation (RNAa) drugs, revolutionary medicines that boost gene function. In April 2024, Nippon Shinyaku, Co., Ltd. (“Nippon Shinyaku”), the parent company of NS Pharma, entered a joint research agreement with MiNA Therapeutics to develop small activating RNAs (saRNAs) to treat rare and intractable diseases of the central nervous system (CNS) by RNAa.
The mission of NS Pharma is to push the boundaries of nucleic acid therapies1 with innovative drug discovery modalities. MiNA Therapeutics shares this goal, using pioneering RNAa medicines to target what was previously deemed “undruggable” by conventional approaches.
MiNA’s proprietary RNAa technology powers efficient saRNA synthesis and screening, while NS Pharma’s pharmacological expertise drives the drug development process. Co-creation combines these strengths, accelerating the delivery of high-quality candidates.
| The Challenge
Around 95% of rare diseases lack approved treatments.2 Conventional approaches, including gene therapy, often fail, particularly when targeting longer genes (>4.7 kb). |
The Strategy
RNAa therapeutics unlock possibilities for targets once deemed untreatable. We’re using this powerful technology to tackle severe neurological disorders. |
The Vision
Collaboration drives unique, effective solutions at unparalleled speed. By combining our strengths, we’re accelerating drug development to help patients sooner. |
Our alliance with MiNA focuses on enhancing gene expression in rare neurological disorders through cutting-edge RNAa therapeutics. The initial target of this collaboration is a severe neurodevelopmental disorder caused by a single defective gene. Although estimated to affect hundreds of thousands of people worldwide, there are currently no approved treatments for this condition.
This disorder is caused by mutations that result in the loss of function of a gene, leading to insufficient levels of the corresponding protein. saRNAs offer a novel way to address this by enhancing the affected gene’s expression, potentially restoring normal function.3 “MiNA’s agility and complementary expertise will enable us to bring this product to the clinic much faster, ultimately helping patients sooner,” Morokata emphasizes.
| 80%
Of rare diseases have a genetic cause2 Around 240 million people worldwide have a rare genetic disorder, with most lacking approved treatments. We’re expediting nucleic acid drug development to close this treatment gap. |
⬆2-10
Fold increase in gene expression4 RNAa therapeutics provide a game-changing opportunity to treat loss-of-function genetic disorders. These drugs restore physiological protein levels by substantially increasing endogenous gene expression. |
⬇50%
Decrease in candidate development timeline The complementary combination of MiNA’s systematic drug discovery approach and NS Pharma’s efficient clinical development process drastically reduces the time needed to produce a clinical candidate. |
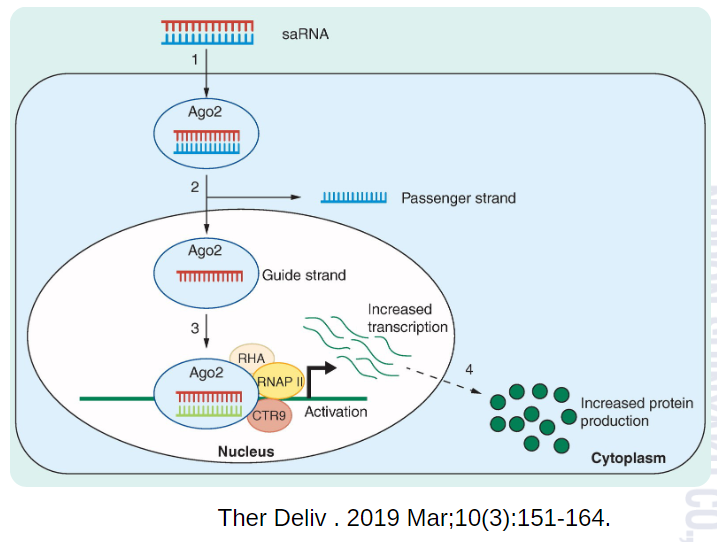
With RNAa technology, we can upregulate genes underlying loss-of-function disorders that cause developmental, motor, and behavioral delays, offering solutions where other therapies have failed.
RNAa therapeutics directly tackle loss-of-function disorders by boosting natural gene expression, increasing it by 2 to 10 times.4 They uniquely activate challenging gene types, including large genes and those silenced epigenetically.
By activating endogenous gene expression, RNAa drugs achieve more physiologically relevant protein expression levels. This mitigates risks associated with overexpression or the permanent introduction of foreign genetic material. The highly specific nature of RNAa therapeutics also minimizes the potential for off-target effects.
Combining NS Pharma’s robust development pipeline with MiNA’s cutting-edge RNAa technology positions us to make significant strides in areas where traditional therapies have struggled.
As we look to the future, our collaboration with MiNA Therapeutics represents more than just a strategic partnership—it embodies our commitment to advancing the treatment of rare diseases through co-creation and innovation.
We are committed to finding solutions for the unmet medical needs of patients worldwide. “By combining our strengths and resources, we can compete with very large pharmaceutical companies, particularly in the rare disease space,” Morokata explains. With our deep expertise and patient-centric focus, we are eager to partner with like-minded organizations to bring groundbreaking treatments to those in need.
Together, we can make a difference.
>Partner with us to advance rare disease treatment<
References